Healthcare & Life Sciences Legal Careers
Healthcare & Life Sciences legal jobs at Axiom let you love the law, love your life with meaningful work and the flexibility to shape your career.


















The work you’ll do as a healthcare & life sciences lawyer at Axiom
As an Axiom healthcare & life sciences lawyer, you'll engage in high-impact work across multiple industries while enjoying the flexibility to shape your career on your own terms, including:
Advising on HIPAA compliance, Stark Law, Anti-Kickback Statute, fraud and abuse laws, and federal and state healthcare regulations. You'll support compliance with Medicare, Medicaid, TRICARE, and 340B Drug Pricing Program requirements, conduct regulatory risk assessments, respond to government investigations and audits, and advise on healthcare provider agreements and payor contracts. Your counsel helps hospitals, health systems, physician practices, and healthcare organizations navigate complex regulatory frameworks while maintaining ethical standards and minimizing compliance risk across operations.
Providing strategic guidance on FDA regulatory submissions, product approval pathways, post-marketing compliance, and adverse event reporting for pharmaceutical companies, medical device manufacturers, and biotechnology firms. You'll advise on regulatory strategy for drug and device development, support inspections and enforcement actions, ensure compliance with Good Clinical Practice and Good Manufacturing Practice standards, and navigate complex federal regulations governing product development and commercialization. Your expertise accelerates the path from concept to market while ensuring regulatory compliance.
Negotiating clinical trial agreements with sponsors, principal investigators, and research institutions while ensuring compliance with IRB requirements and federal regulations. You'll draft informed consent forms, manage multi-site clinical research contracts, advise on data ownership and publication rights, coordinate collaborations between pharmaceutical companies and academic medical centers, and protect intellectual property and confidential information throughout the research process. Your work supports Phase I-IV clinical trials and investigator-initiated research while balancing legal precision with operational efficiency.
Drafting and negotiating manufacturing services agreements, commercial supply agreements, and CDMO contracts for pharmaceutical and biotechnology companies. You'll advise on cGMP manufacturing requirements, establish quality agreements and specifications, manage raw materials sourcing and packaging arrangements, negotiate contract manufacturing and toll manufacturing deals, and address warehousing and logistics considerations. Your counsel ensures reliable supply chains while protecting intellectual property, managing performance metrics, and maintaining regulatory compliance across complex manufacturing relationships.
Advising on government pricing programs including Medicare, Medicaid, VA Federal Supply Schedule, and 340B Drug Pricing Program compliance. You'll negotiate commercial payer contracts and PBM agreements, develop value-based contracting arrangements, support drug price reporting obligations including AMP and Best Price calculations, advise on Inflation Reduction Act compliance and CMS negotiations, and structure patient access programs and affordability initiatives. Your strategic guidance navigates the complex pharmaceutical supply chain while optimizing market access and ensuring compliance with pricing regulations across federal and commercial channels.
Negotiating physician employment agreements, healthcare provider service agreements, payor and managed care contracts, and health information technology platform agreements. You'll support M&A due diligence for healthcare and life sciences transactions, draft collaboration and licensing agreements, advise on telehealth and digital health legal frameworks, and handle commercial relationships across the healthcare ecosystem. Your work helps clients structure transactions and partnerships that advance innovation while managing regulatory and commercial risks.

of Axiom legal talent would refer their peers to Axiom
Healthcare & Life Sciences Lawyers & Legal Professionals
Axiom legal talent are satisfied with their engagement
Healthcare & Life Sciences Law Engagements
Why become an Axiom healthcare & life sciences lawyer?
- Advise on healthcare regulatory compliance, FDA matters, clinical trials, manufacturing, and market access for 75% of the Fortune 100
- Join less than 3% of applicants accepted, collaborating with legal professionals averaging 18-20+ years of experience
- 93% of clients rate Axiom lawyers as good as or better than top law firm attorneys
- Design your career with dedicated 1:1 support from your Talent Success Partner focusing on engagement success and development
- Choose engagements aligned with your goals—regulatory compliance, clinical trials, market access, manufacturing—building a dynamic, multifaceted career
- Access legal AI, accredited CLEs from PLI and LexisNexis, plus coaching that prepares you for wherever your career takes you
- Choose where, when, and how you work—full-time or part-time, with flexible arrangements including remote, on-site, or hybrid options
- Work as many or as few hours as you'd like, taking time between assignments when it suits you
- Join a culture that invests in your success: over Over 20% of our professionals have been with Axiom for more than four years, building long-term careers with us

Working at Axiom
Frequently Asked Questions
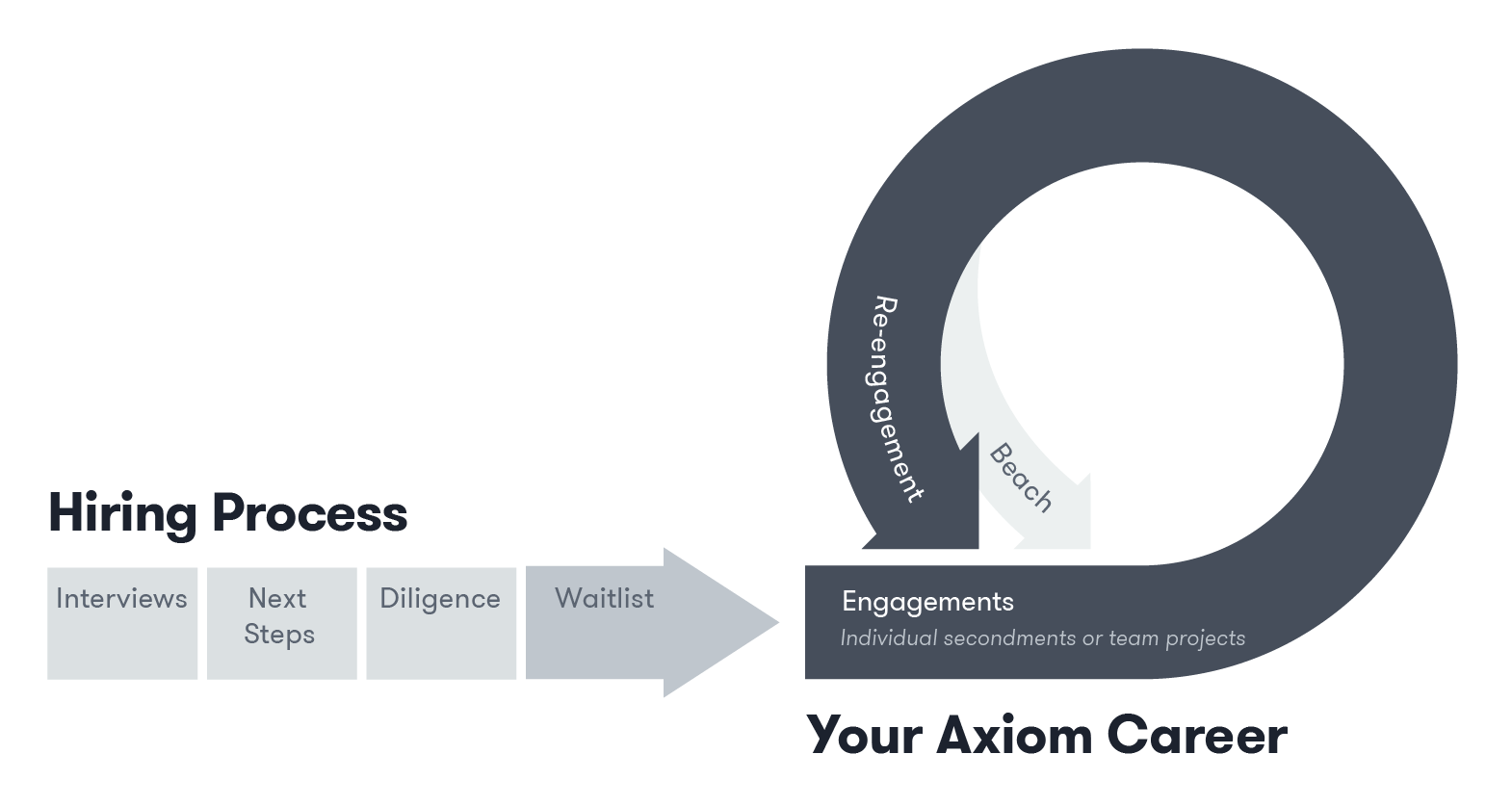
Axiom’s interview process typically takes about two weeks. If your interviews are successful, you'll work on your Axiom for Talent profile while our recruiting team conducts diligence checks. Once complete, you'll be introduced to your Talent Success Partner and join our Waitlist.
After you’ve matched with a client engagement and completed our onboarding process, you become an Axiom employee. Your Talent Success Partner supports you throughout your engagement and helps you find your next role while you're still working—or you can spend time "on the beach" between engagements.
Yes! While offerings vary by region, many of our offices organize social events, discussion groups, guest speakers, and activities. You'll also have access to Axiom's Employee Resource Groups (ERGs), including DNA (representing our Disability, Neurodiversity & Allies community), ERA (Equal Rights at Axiom - representing women at Axiom), Apaxiom (representing our AAPI community), Blaxiom (representing our Black community), and Outlaws (representing our LGBTQIA+ community).
Beyond formal events, you'll be part of a vibrant community of high-caliber legal professionals, and we leverage this impressive network through mentoring programs and opportunities to share best practices and collaborate with colleagues across our global platform.
The average engagement lasts approximately eight months globally, though many legal talent stay engaged with their clients for much longer—sometimes years. About 4-6 weeks before your engagement concludes, your Talent Success Partner will begin discussing your next opportunity and works to find your next engagement while you're still engaged.
Between engagements (what we call being “on the beach”), you'll have time to pursue your interests and goals outside of Axiom; you’ll continue to have access to our professional development resources. Our legal talent’s utilization rate is around 70%, meaning legal talent are working as many (or as few) hours as they'd like.